Main functions
Chlorophyll fluorescence and P700 are measured individually or simultaneously
Induction kinetic curves of two optical systems (including fast and slow phases)
Fast light curve and light response curve for two optical systems
Quenching analysis, dark relaxation analysis
Typical P700 curve measurements
Through synchronous measurement of chlorophyll fluorescence and P700, the electron transfer kinetics of the two optical systems, the size of the electron carrier library, the ring electron transfer kinetics surrounding PSI, etc.
Measurement parameters
PS II parameters: Fo, Fm, F, Fm’, Fv/Fm, Y(II) i.e.△F/Fm’, Fo’, qP, qL, qN, NPQ, Y(NPQ), Y(NO) and ETR(II), etc.
PS I parameters: P700, Pm, Pm’, P700red, Y(I), Y(ND), Y(NA) and ETR(I), etc.
Other measurement parameters: Post-Illumination (drum), PQ-Pool (PQ library), etc.
Application areas
Especially suitable for in-depth PSII and PSI activity measurements in the field, it is a powerful assistant in plant physiology, plant ecology, agronomy, forestry, horticulture, and plant adversity research.The fiber version is designed to be lighter and easy to carry. In addition, the fiber version is especially suitable for in-situ measurement of samples such as moss and lichens.
Main technical parameters
P700 Dual wavelength measurement light: LED, 830 nm and 875 nm
PSII fluorescence measurement light: LED, 460 nm or 620 nm
Red actinic light: LED array, 635 nm; maximum continuous light intensity 4000 μmol m-2yes-1
Blue photoelectric light: LED, 460 nm; maximum continuous light intensity 500 μmol m-2yes-1
Single turnover saturated flash (ST): 200000 μmol m-2yes-1, 5~50 μs adjustable
Multi-turnover saturated flash (MT): 20000 μmol m-2yes-1, 1~1000 ms adjustable
Far Red Light: 720 nm
Purchase Guide
1. Basic styles of higher plants leaves
System composition: fiber version host, fiber optic, light adaptive leaf clip, dark adaptive leaf clip, software, etc.
Note: Portable fiber-optic dual-channel modulated chlorophyll fluorescence instrument has both red and blue light
 |
| Dual-PAM/F Basic |
2. Basic hanging sample measurement
System composition: general-purpose host, optical fiber, sample pool for suspension measurement, software, etc.
Note: When purchasing suspended samples to measure basic models, you can not choose a light adaptive leaf clip. It is recommended to choose a magnetic stirrer.
 |
| Dual-PAM/F basic suspension sample measurement model |
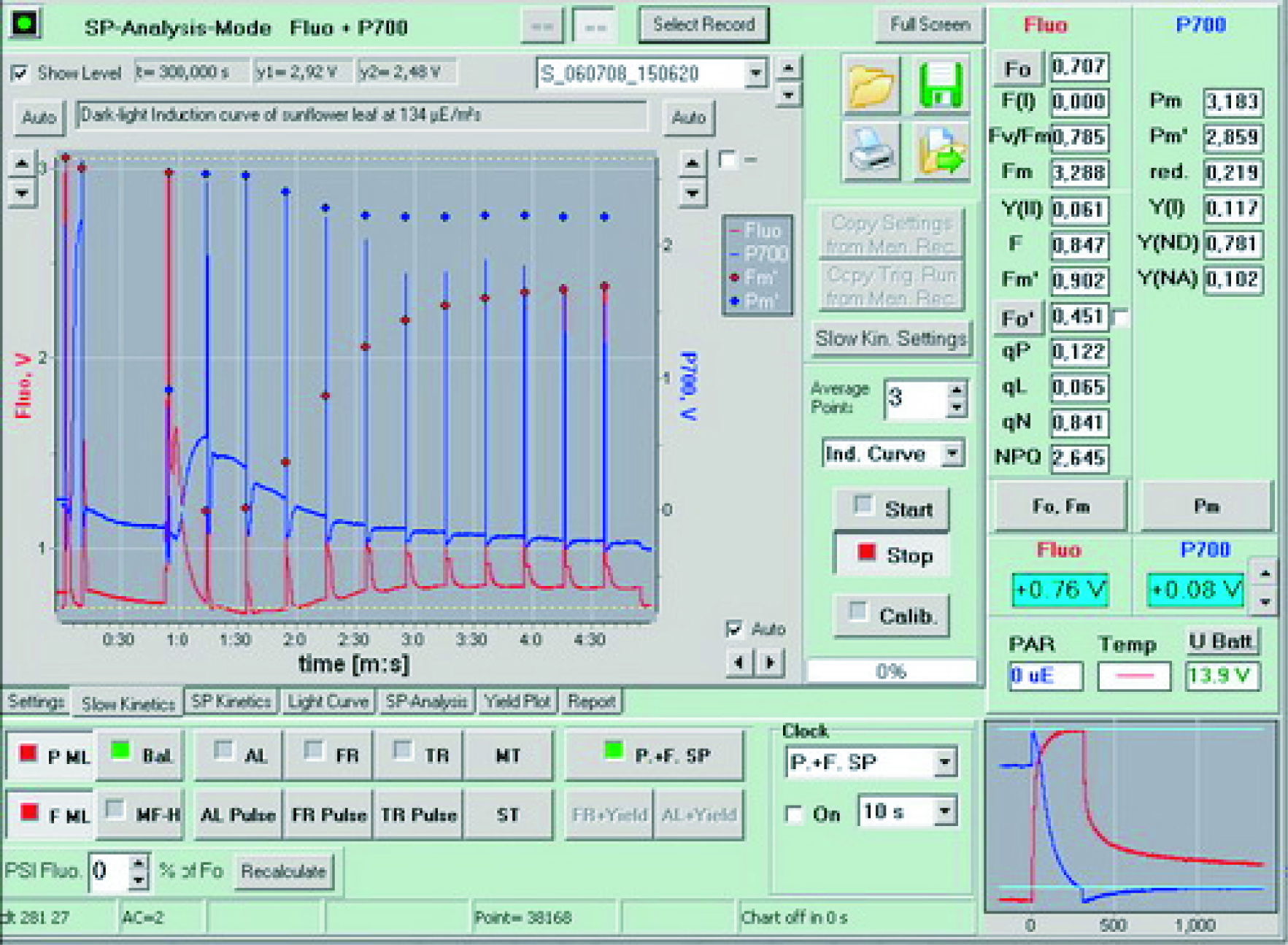 | 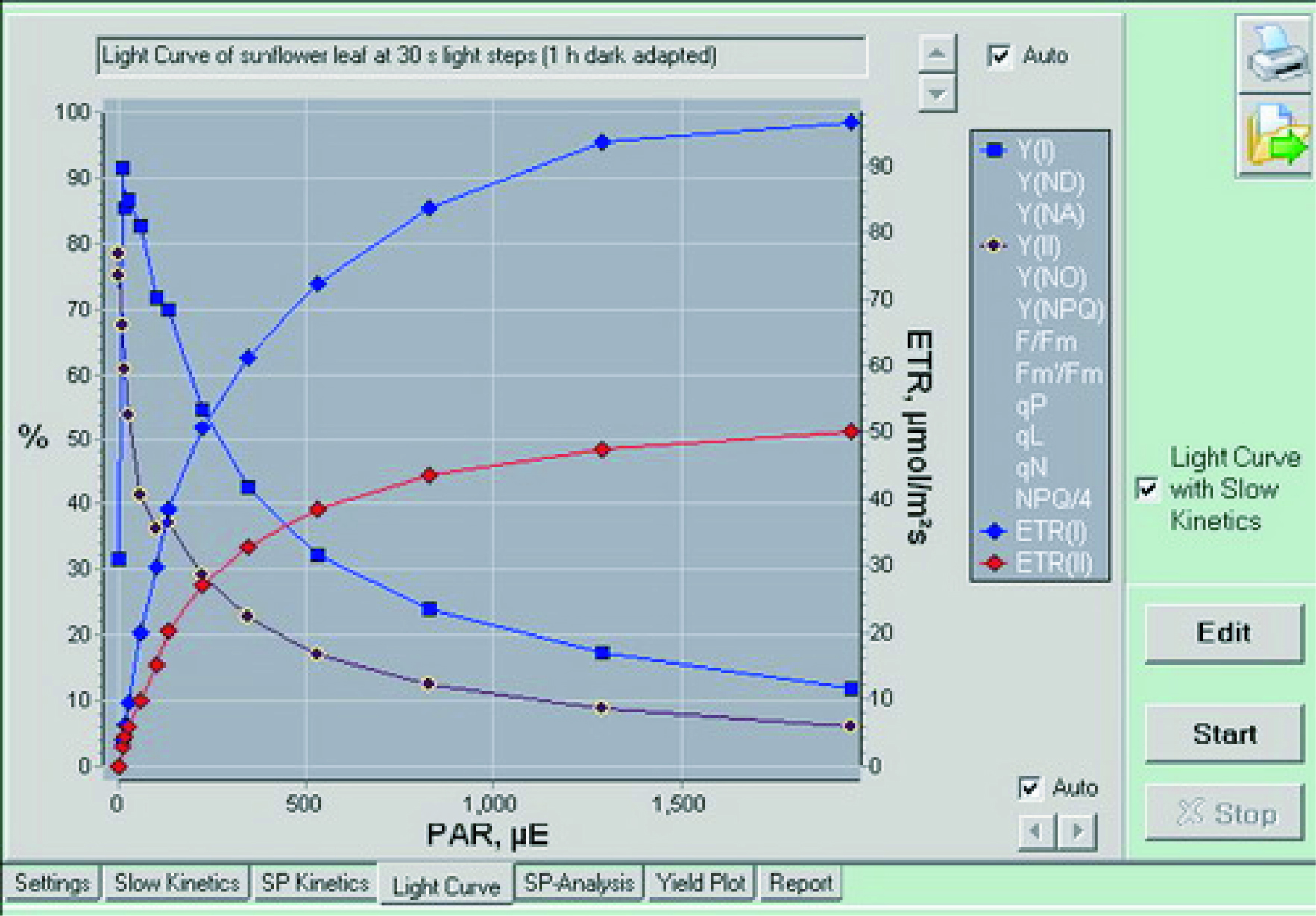 | 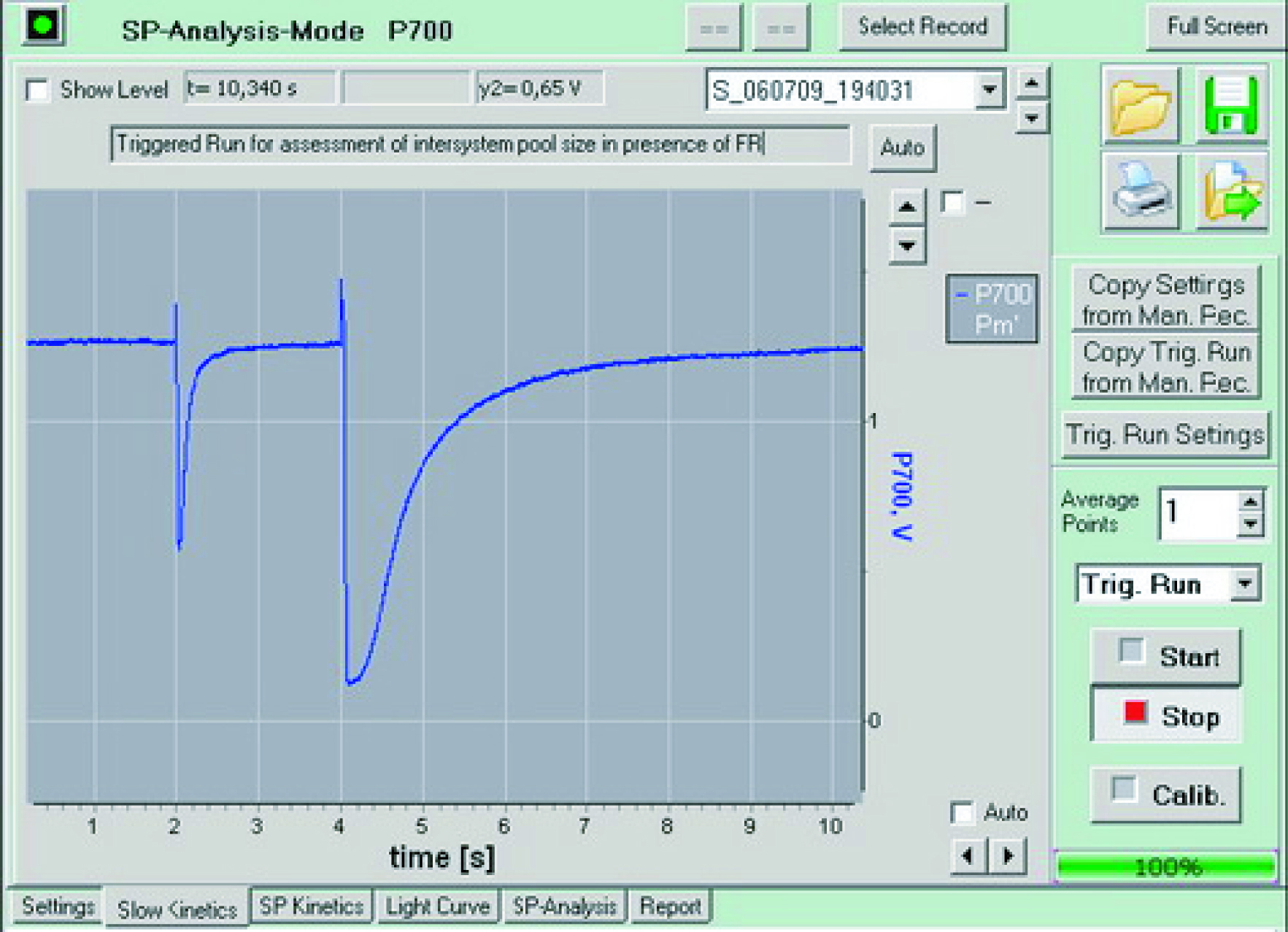 |
| Synchronous measurement PSII (red) and PSI (blue) induction curve | Synchronous measurement PSII (red) and PSI (blue) light response curve | Typical P700 measurement curve |
 |  |  |
| chlorophyll fluorescence signal when the saturation pulse is turned on (red) and P700 (blue) Signal changes | Fluorescence rapid kinetic curve measured in linear time | Fluorescence rapid kinetic curve measured in logarithmic time |
3. Other optional accessories
1, 2060-B: Arabidopsis leaf clip, 60-degree angle light adaptive leaf clip, used in conjunction with independent micro-photo quantum/temperature sensor 2060-M, especially suitable for measuring Arabidopsis leaflets.The prerequisite for use is to configure 2060-M.
2, 2060-M: Miniature photo quantum/temperature sensor, measuring PAR and temperature, can be connected to MINI-PAM and used independently, mostly combined with 2060-B.
3. MKS-2500: A magnetic stirrer configured for KS-2500, specially configured for KS-2500, is installed under the KS-2500, which drives the rotor inside the KS-2500 to stir the liquid sample.
4, 2030-B90: 90-degree angle fiber adapter, mounted on 2030-B or 2060-B, bringing the fiber to a 90-degree angle to the sample.
Origin: WALZ, Germany
References
Data source: Photosynthesis Literature Endnote database, updated to September 2016, with more than 6,000 documents
Original data source: Google Scholar
Zhou, W., et al. (2016). "Effects of sodium bicarbonate concentration on growth, photosynthesis, and carbonic anhydrase activity of macroalgae Gracilariopsis lemaneiformis, Gracilaria vermiculophylla, and Gracilaria chouae (Gracialiales, Rhodophyta)." Photosynthesis Research: 1-12.
Yamori, W., et al. (2016). "A physical role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice." Scientific Reports 6.
Yamamoto, H., ETA. (2016). "artificial Re model of alternative electron flow by fla VOIron protein Sina rabid op sis." nature plants 2: 16012.
Wang, H., et al. (2016). "The sporulation of the green alga Ulva prolifera is controlled by changes in photosynthesis electron transport chain." Scientific Reports 6: 24923.
X UE, X., ETA. (2016). "development of the photosynthesis apparatus of Cunningham i Alan CEO sloppy in light and darkness." new pH should be mentioned by log i: You/ah-you/ah.
Shimakawa, G., et al. (2016). "Diversity in photosynthesis electron transport under [CO2]-limitation: the cyanobacterium Synechococcus sp. PCC 7002 and green alga Chlamydomonas reinhardtii drive an O2-dependent alternative electron flow and non-photochemical quenchingof chlorophyll fluorescience during CO2-limited photosynthesis." Photosynthesis Research: 1-13.
Tadini, L., et al. (2016). "GUN1 controls accumulation of the plastid ribosomal protein S1 at the protein level and interacts with proteins involved in plastid protein homeostasis." Plant Physiology: pp. 02033.02015.
Takagi, D., et al. (2016). "Photorespiration provides the chance of cyclic electron flow to operate for the redox-regulation of P700 in photosynthesis electron transport system of sunflower leaves." Photosynthesis Research: 1-12.
Leonelli, L., et al. (2016). "Transient expression in Nicotiana benthamiana for rapid functional analysis of genes involved in non-photochemical quenching and carotenoid biosystemthesis." The Plant Journal: n/a-n/a.
men eg and SSO, A., ETA. (2016). "photo acclimation of photosynthesis int and EU stigma top Heinze Ann press no or op sis GA carpet A." photosynthesis research: 1-15.
Huang, W., et al. (2016). "PSI photoinhibition is more related to electron transfer from PSII to PSI rather than PSI redox state in Psychotria rubra." Photosynthesis Research: 1-8.
Mishanin, V. I., et al. (2016). "Light acclimation of shade-tolerant and light-resistant Tradescantia species: induction of chlorophyll a fluorescence and P700 photooxidation, expression of PsbS and Lhcb1 proteins." Photosynthesis Research.
Benson, S. L., et al. (2015). "An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis." Nature Plants 1: 15176.
Gao, F., et al. (2015). "NdhV Is a Subunit of NADPH Dehydrogenase Essential for Cyclic Electron Transport in Synechocystis sp. Strain PCC 6803." Plant Physiology: pp. 01430.02015.
Gerotto, C., et al. (2015). "In Vivo Identification of Photosystem II Light Harvesting Complexes Interacting with PHOTOSYSTEM II SUBUNIT S." Plant Physiology 168(4): 1747-1761.
Giovagnetti, V., et al. (2015). "Assessment of the impact of photosystem I chlorophyll fluorescience on the pulse-amplitude modulated quenching analysis in leaves of Arabidopsis thaliana." Photosynthesis Research: 1-11.
Iwai, M., et al. (2015). "Light-harvesting complex Lhcb9 confers a green alga-type photosystem I supercomplex to the moss Physcomitrella patens." Nature Plants 1(2).
Timm, S., et al. (2015). "Mitochondrial Dihydrolipoyl Dehydrogenase Activity Shapes Photosynthesis and Photorespiration of Arabidopsis thaliana." The Plant Cell: tpc. 15.00105.
Tsabari, O., et al. (2015). "Differential effects of ambient or diminished CO2 and O2 levels on thylakoid membrane structure in light‐stressed plants." The Plant Journal 81(6): 884-894.
Zhao, J., et al. (2015). "NdhQ Is Required to Stabilize the Large Complex of NADPH Dehydrogenase in Synechocystis sp. Strain PCC 6803." Plant Physiology: pp. 00503.02015.
Zivcak, M., et al. (2015). "Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves." Photosynthesis Research: 1-15.